Bristol-Myers Squibb Company obtained approvals of 18 new drugs and additional drug indications and formulations in 2022.
These drugs are currently marketed in the company’s main markets, the United States and Japan.
With 34,300 employees in 44 countries, Bristol-Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients overcome serious diseases.
Among its advances in oncology, the U.S. Food and Drug Administration (FDA) and the European Commission (EC) approved Opdualag, the first combination PD-1 inhibitor and LAG-3 blocking antibody.
In addition, in the United States, the FDA and the EC approved the first PD-1 inhibitor and LAG-3 blocking antibody.
The US, EU and Japan approved two Opdivo-based regimens as first-line treatments for advanced or unresectable metastatic ESCC.
The company continues to advance and invest in its cell therapy portfolio through the approval of Abecma in Japan for the treatment of multiple myeloma in patients with at least three prior therapies, and approvals of Breyanzi for relapsed or refractory diffuse large B-cell lymphoma, with second-line treatments in the United States and Japan, and third-line treatments in the EU.
Bristol-Myers Squibb also continues to expand its cell therapy manufacturing capabilities at its existing facilities in Washington and New Jersey, as well as through the construction of new state-of-the-art manufacturing facilities in Massachusetts and Leiden, the Netherlands.
New drugs
The approvals of Sotyktu (deucravacitinib) in the United States and Japan for the treatment of moderate to severe plaque psoriasis expanded its portfolio in immunology.
In cardiovascular, the company expanded its pipeline of new products with the FDA approval of Camzyos (mavacamten) for patients with symptomatic obstructive HCM.
In addition, in August 2022, it acquired Turning Point, a precision oncology company, with the aim of expanding its solid tumor portfolio with the addition of repotrectinib.
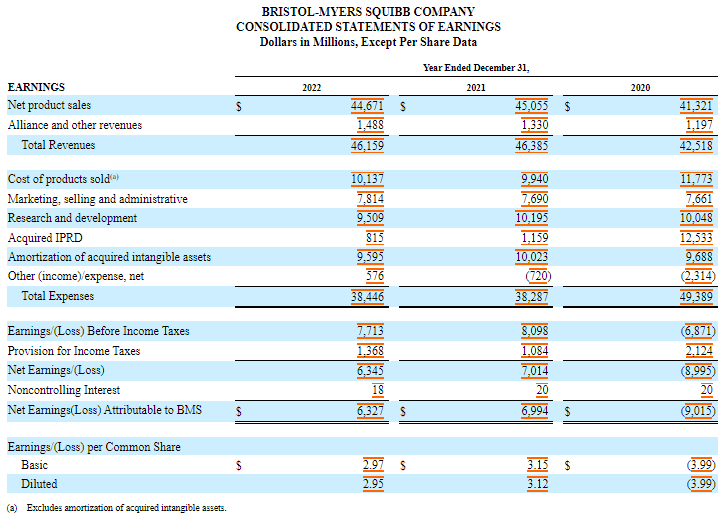
Its R&D spending totaled $9.5 billion in 2022, $10.2 billion in 2021 and $10 billion in 2020.
Acquired ongoing research and development (IPRD) expenses were $815 million, $1.2 billion and $12.5 billion in 2022, 2021 and 2020, respectively.
In 2020, the acquired IPRDs included a charge of US$11.4 billion resulting from the acquisition of MyoKardia.
![]()

